Abstract

Introduction:
Hepatitis B core antibodies (Anti-HBC+) are virtually detected in all patients who have been exposed to Hepatitis B Virus (HBV), which make it a reliable serology marker for the previous infection. Anti-HBC+ patients undergoing hematopoietic stem cell transplant (HSCT) are at high risk of reactivation and may change the outcome especially during immunosuppression therapy (IST) phase which believed to enhance viral replications, as with withdrawal of IST which lead to the restoration of immune function making infected hepatocyte easier to destruction. The exact risk of (Anti-HBC+) post HSCT is not very well studied. In this study, we attempted to demonstrate the effect of (Anti-HBC+) positivity in the outcome post HSCT.
Methods:
Patients who were at least 14 years old receiving HSCT between year 2004 and 2010 at our center were reviewed. Patients with acutely infected hepatitis B (+ve hepatitis S antigen and IgM anti-HBC) were excluded from HSCT. A total of 489 patients were identified and included in the study. Patients were stratified into two groups: anti-hepatitis B core serology positive (Anti-HBC+) versus anti-hepatitis B core serology negative (anti-HBC -ve). After transplant, hepatitis B serology and PCR testing were performed at physician discretion at the time of unexplained elevation of liver enzymes. Prophylaxis use of antiviral medications was routinely given to all patients who are anti-HBC positive. Interpretation of hepatitis B serological test was based on Center for Disease Control and Prevention (CDC).
Results:
Median age for all patients is 22 years (range, 14 -62), 62% (n=304) were males. Matched related donor was used for majority of patients, 94.3% (n=461), while 18 patients (3.7%) underwent mismatched HSCT and 7 patients (1.4%) had umbilical cord transplantation. Diagnoses were ALL in 158 patients (32%), AML in 142 (29%) and CML in 58 patients (12%).GVHD prophylaxis was CSA/MTX for 83% (n=405), CSA/MMF for 4% and others for 13% (n=63). Conditioning regimen was Cyclophosphamide/TBI in 201 (41%), Busulfan/Cyclophosphamide in 181 patients (37%), Fludarabine/cyclophosphamide in 52 patients (11%) and others in 54 patients (11%). Bone marrow was the source of stem cells for 288 patients (59%).
Anti-HBC serology at the time of HSCT was positive in 76 patients (15.5%) versus negative in 413 patients (84.5%). Donors' anti-HBC status was positive in 14% versus negative in (86%).
A median follow-up of 5.4 years for anti-HBC+ survivors (range, 3 -5) showed 31 patients (41%) remain positive, 39 patients (51%) sero-converted to negative and six patients (8%) developed re-activation of hepatitis B. A median follow-up unreached for anti-HBC -ve survivors showed 396 patients (95.9%) remain negative, 6 patients (1.9%) became positive for anti-HBC and 3 patients (1%) developed acute hepatitis B after HSCT.
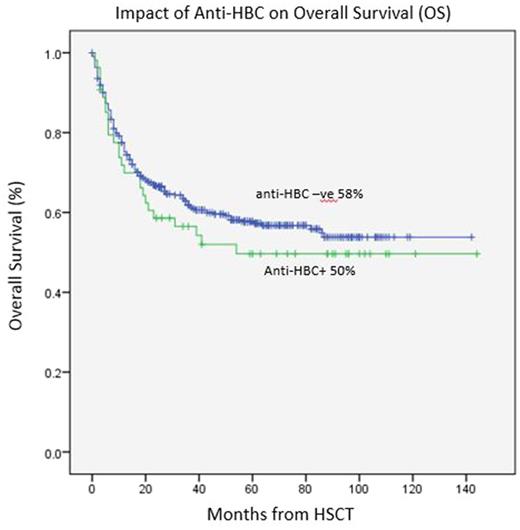
Outcomes of HSCT showed overall survival (OS) rate at 5 years of 50% in anti-HBC+ group versus 58% in anti-HBC -ve group (p=0.30), suggesting a trend of lower rate of OS in anti-HBC+ group. The NRM and cumulative incidence of relapse at 5 years was 19% and 42% in anti-HBC+ group versus 17% (p=0.9) and 35% in anti-HBC -ve group (p=0.20), respectively.
The cumulative incidence of overall acute GVHD at day 120 and overall chronic GVHD at 2 years were 42% and 47% in anti-HBC+ group versus 33% (p=0.1) and 46% (p=0.9) in anti-HBC -ve group, respectively, suggesting a trend of higher incidence of overall acute GVHD in anti-HBC+ group however it was statistically insignificant.
Univariate analysis of risk factors for OS showed anti-HBC+ pre HSCT is an important factor however this was not confirmed in multivariate analysis.
Conclusion:
The present study suggested very low incidence of hepatitis B re-activation following HSCT for anti-HBC+ group (8%) if given proper anti HBV prophylaxis. Anti-HBC+ group does have a trend of lower overall survival and higher association with overall acute GVHD however this was not confirmed in multivariate analysis. Further studies are strongly warranted to confirm the prognostic role of anti-HBC in HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal